When you hear the word "biosimilar," you might think it’s just another word for generic. But that’s not true-especially when it comes to monoclonal antibody biosimilars. These aren’t simple chemical copies like the pills you pick up at the pharmacy. They’re complex, living medicines made from living cells, designed to mirror the exact behavior of their original biologic counterparts. And they’re changing how we treat cancer, autoimmune diseases, and other serious conditions.
What Makes Monoclonal Antibody Biosimilars Different?
Think of a monoclonal antibody as a custom-built key. It’s made to fit one specific lock in your body-like a protein that tells cancer cells to grow or an immune system signal that causes inflammation. The original version, called the reference product, is developed over years, with exacting controls over how the cells are grown, how the protein is folded, and how sugars (called glycans) are attached to it. These tiny changes affect how the drug works and how safe it is.
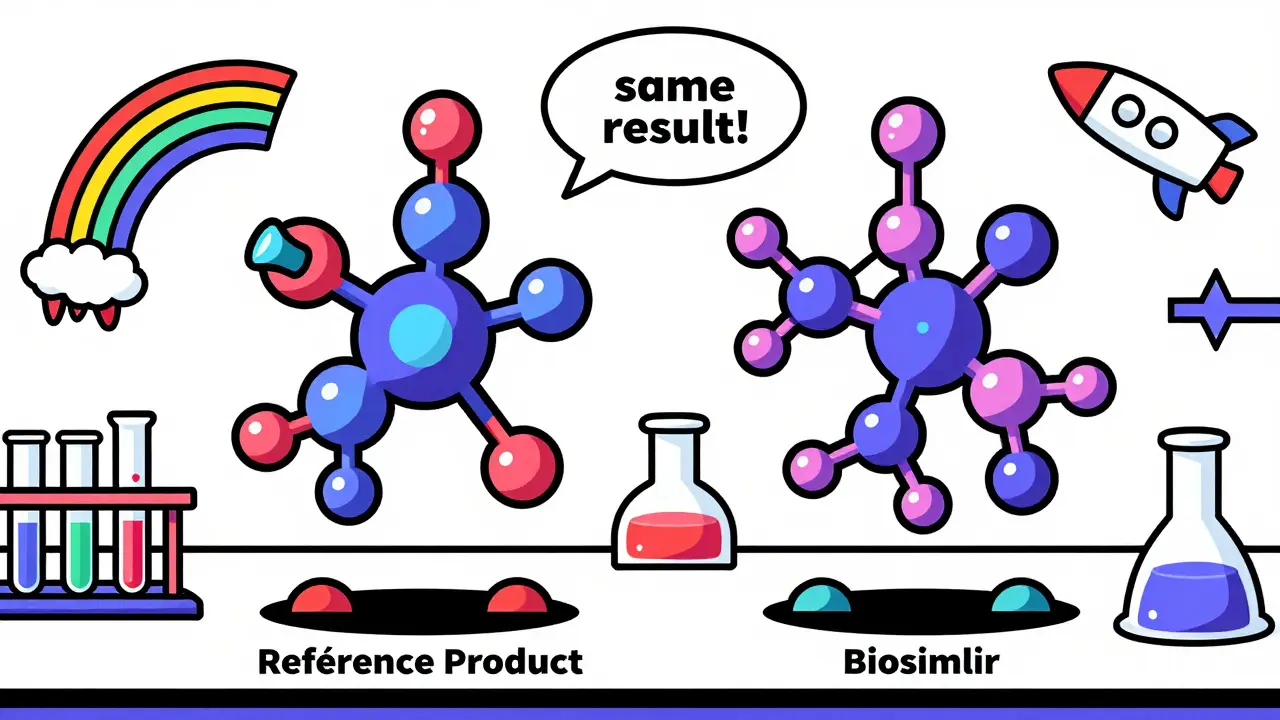
A biosimilar doesn’t copy the original molecule exactly. It can’t. Even the same company making the same drug twice can’t get it to be 100% identical. That’s because biological molecules are made by living cells, and those cells don’t follow a blueprint like a machine does. They wiggle, they vary, they respond to temperature, nutrients, and even the air in the room. So biosimilar makers don’t try to clone the original. Instead, they build a molecule that behaves the same way-binding to the same target, triggering the same immune response, and breaking down at the same rate.
The FDA says a biosimilar must have no clinically meaningful differences in safety, purity, or potency compared to the reference product. That’s not a vague promise. It means hundreds of tests-analytical, animal, and human trials-must prove the biosimilar acts like the original in every way that matters to patients.
How Are They Approved? It’s Not Like Generics
Generic drugs are chemically identical to their brand-name versions. You can dissolve them, test the atoms, and see the same structure every time. Biosimilars? Not even close. A small molecule like aspirin weighs about 180 daltons. A monoclonal antibody? Around 150,000 daltons. That’s like comparing a bicycle to a jet fighter. You can’t just reverse-engineer it.
So the approval path is longer and tougher. Developers must:
- Perform hundreds of lab tests comparing the structure, charge, shape, and sugar attachments of the biosimilar to the original
- Run animal studies to confirm similar biological activity
- Conduct clinical trials in healthy volunteers and patients to prove safety and effectiveness
- Focus on the most sensitive use case-the one most likely to reveal any difference
For example, if a biosimilar of rituximab is being made, regulators will test it in rheumatoid arthritis patients first, because their immune response is highly sensitive to small changes in the antibody’s structure. If it works just as well there, it’s likely to work in lymphoma too.
Approved Monoclonal Antibody Biosimilars and What They Treat
As of 2026, more than 20 monoclonal antibody biosimilars have been approved in the U.S. alone. Here are the major ones and what they’re used for:
Bevacizumab Biosimilars (Avastin)
Bevacizumab blocks a protein that helps tumors grow new blood vessels. It’s used for colorectal, lung, ovarian, and brain cancers. Six biosimilars are approved:
- Mvasi (2017)
- Zirabev (2019)
- Alymsys (2019)
- Vegzelma (2022)
- Avzivi (2023)
- Jobevne (2023)
Studies show these biosimilars work just as well as Avastin, with no increase in side effects like high blood pressure or bleeding.
Trastuzumab Biosimilars (Herceptin)
Trastuzumab targets HER2, a protein overexpressed in 15-20% of breast cancers and some stomach cancers. Six biosimilars are approved:
- Ogivri (2017)
- Herzuma (2018)
- Ontruzant (2019)
- Trazimera (2020)
- Kanjinti (2019)
- Hercessi (2022)
A 2021 trial comparing Ogivri to Herceptin in over 500 women with early-stage breast cancer found identical rates of tumor shrinkage and survival. No difference in heart damage-a major concern with this drug.
Rituximab Biosimilars (Rituxan)
Rituximab wipes out certain immune cells, making it useful for non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, and autoimmune diseases like rheumatoid arthritis. Three biosimilars are approved:
- Truxima (2018)
- Riabni (2020)
- Ruxience (2020)
A 2022 study in JAMA Oncology tracked 1,247 patients at 15 U.S. cancer centers who switched from Rituxan to Truxima. The cost dropped 28% per cycle. No increase in infections, infusion reactions, or disease flare-ups.
Adalimumab Biosimilars (Humira)
Adalimumab blocks TNF-alpha, a key driver of inflammation in rheumatoid arthritis, Crohn’s disease, psoriasis, and more. Over a dozen biosimilars have been approved since 2023, including Hyrimoz, Hadlima, and Amjevita. These have replaced Humira in many clinics, cutting monthly costs from over $2,000 to under $500.
Cost Savings Are Real-And Massive
Biologics used to cost $100,000+ per year. A single dose of Herceptin could run $5,000. Biosimilars cut those prices by 30-70%.
Analysts at Evaluate Pharma predict biosimilar monoclonal antibodies will save the U.S. healthcare system $250 billion between 2023 and 2028. Bevacizumab, trastuzumab, and rituximab biosimilars will account for 78% of those savings.
One reason? Competition. When three or four biosimilars hit the market for one drug, prices tumble. For example, the average price of trastuzumab biosimilars dropped 58% in the first year after launch.
Interchangeable Biosimilars: The Next Step
Not all biosimilars can be swapped in and out like a generic pill. To be called "interchangeable," a biosimilar must prove that switching back and forth between it and the original causes no extra risk. That’s a higher bar.
In July 2023, Celltrion’s Remsima (infliximab biosimilar) became the first monoclonal antibody biosimilar to earn this designation from the FDA. That means pharmacists can substitute it for the original without needing the doctor’s OK-just like with aspirin.
More interchangeable biosimilars are expected by 2026, especially for adalimumab and rituximab. This will speed up adoption in community pharmacies and reduce administrative delays.

Challenges Still Exist
Despite the progress, hurdles remain.
- Patent battles: Drugmakers fight to delay biosimilars. The average monoclonal antibody biosimilar faces 14.7 patent lawsuits before launch.
- Provider hesitation: A 2022 ASCO survey found only 58% of oncologists felt "very confident" prescribing biosimilars. Many still worry about subtle immune reactions.
- Formulary barriers: Pharmacy benefit managers sometimes block biosimilars to protect revenue from the original brand.
And while rare, immune reactions do happen. The EMA reported 12 cases of unexpected immune responses across 1.2 million patient-years of biosimilar exposure. But that rate was the same as the reference products-meaning the biosimilars weren’t causing more problems.
What’s Next?
The pipeline is full. As of 2023, 37 monoclonal antibody biosimilars were in FDA review. The biggest focus? Pembrolizumab (Keytruda) biosimilars-six are in late-stage trials. Keytruda alone brought in over $20 billion in 2023. Once biosimilars arrive, prices could drop by 80%.
The EMA plans to release new guidelines in 2024 for even more complex biologics: bispecific antibodies and antibody-drug conjugates. These are next-gen drugs that carry chemotherapy directly to cancer cells. Making biosimilars of these will be even harder-but the payoff? Huge.
By 2027, IQVIA predicts monoclonal antibody biosimilars will make up 35% of all biologic prescriptions in the U.S.-up from 18% in 2022. Cancer treatments will drive 62% of that growth.
Bottom Line
Monoclonal antibody biosimilars aren’t just cheaper versions. They’re scientifically validated, clinically proven alternatives that deliver the same results at a fraction of the cost. They’ve already changed treatment for breast cancer, lymphoma, and autoimmune diseases. And they’re just getting started. The future of biologic therapy isn’t about exclusivity-it’s about access. And biosimilars are making sure more patients get the life-saving treatments they need.
Are monoclonal antibody biosimilars the same as generics?
No. Generics are chemically identical copies of small-molecule drugs, like aspirin or metformin. Biosimilars are highly similar but not identical copies of complex biological drugs made from living cells. Monoclonal antibodies are large proteins with intricate structures and sugar attachments that can’t be perfectly replicated. Biosimilars must prove they work the same way in the body, but they’re not exact copies.
Are biosimilars safe?
Yes. Every biosimilar approved by the FDA or EMA must show no clinically meaningful differences in safety, purity, or potency compared to the original. Thousands of patients have used them, and studies consistently show similar rates of side effects, infections, and immune reactions. Rare immune responses have occurred, but at the same rate as the reference product-meaning the biosimilar isn’t the cause.
Can I switch from the original drug to a biosimilar?
Yes, and many patients already have. For non-interchangeable biosimilars, your doctor must prescribe the biosimilar by name. For interchangeable biosimilars, your pharmacist can substitute it without asking your doctor-just like with a generic pill. Clinical studies confirm switching doesn’t reduce effectiveness or increase side effects.
Why are biosimilars cheaper?
They don’t need to repeat the full clinical trials that the original drug did. Instead, manufacturers prove similarity through analytical testing and targeted studies. This cuts development costs by 60-80%. Plus, competition from multiple biosimilars drives prices down. For example, trastuzumab biosimilars cut the price from $5,000 per dose to under $2,000.
Will biosimilars replace the original drugs entirely?
Not entirely, but they’re taking over. In markets like Europe, where biosimilars have been available longer, over 80% of new prescriptions for drugs like adalimumab are now biosimilars. In the U.S., adoption is growing fast. By 2027, biosimilars are expected to make up 35% of all biologic prescriptions. The original drugs will still be used, especially in cases where patients have been stable on them for years-but new patients are increasingly starting on biosimilars.
Monoclonal antibody biosimilars are no longer the future-they’re the present. They’re saving lives and billions of dollars, one precise, scientifically validated dose at a time.