Have you ever looked at your pharmacy receipt and wondered why the generic version of your medication costs a fraction of the brand-name one? You’re not alone. A pill that costs $500 as a brand-name drug might only set you back $4 as a generic. That’s not a typo. It’s not a scam. And it’s not because the generic is weaker. It’s because of how drugs are developed, approved, and sold.
Same medicine, different price tag
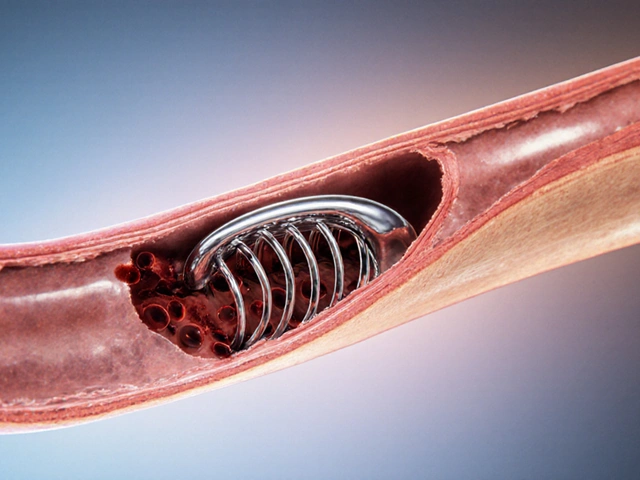
Generic drugs aren’t knockoffs. They’re exact copies of brand-name drugs in every way that matters: the same active ingredient, the same strength, the same way they work in your body. The FDA requires them to deliver the same amount of medicine into your bloodstream at the same rate. That’s called bioequivalence. If your doctor prescribes Lipitor for cholesterol, the generic atorvastatin does the exact same thing. Same effect. Same risks. Same benefits. So why the huge price difference? It all comes down to who pays for what - and who doesn’t have to.The $2.6 billion price of being first
When a pharmaceutical company invents a new drug, they spend over a decade and roughly $2.6 billion to get it to market. That’s not just lab work. It’s hundreds of clinical trials involving thousands of patients. It’s years of animal testing. It’s legal fees, regulatory submissions, and marketing campaigns before a single pill is even sold. That cost gets baked into the price of the brand-name drug. That’s why brand-name companies get patents. The patent gives them 20 years of exclusive rights to sell the drug. That’s their chance to make back their investment - and then some. During those 20 years, no one else can legally make the same drug. That’s monopoly pricing.How generics skip the $2.6 billion bill
Once the patent runs out, other companies can step in. But they don’t start from scratch. They don’t need to repeat all those expensive clinical trials. Instead, they file what’s called an Abbreviated New Drug Application (ANDA). All they have to prove is that their version is bioequivalent to the brand-name drug. That means testing blood levels, absorption rates, and how the body processes the medicine. It’s still rigorous. But it’s not 10 years of trials. It’s months. The cost to develop a generic? Around $1 to $5 million. That’s less than 0.2% of what it cost the original company. No need to fund massive clinical studies. No need to build global marketing campaigns. No need to pay for years of patent litigation. The generic manufacturer just needs to make the pill, prove it works the same way, and get FDA approval.
Competition drives prices down
Once the first generic hits the market, others follow. Within a year, you might see five, ten, even 14 different companies selling the same generic drug. That’s when prices really drop. Companies compete on price. They slash margins. They offer discounts to pharmacies and insurers. The result? A 80-90% price drop, according to the Congressional Budget Office. Take omeprazole. As Prilosec, it cost $300 a month. Now, as a generic, it’s $6. Atorvastatin (Lipitor) went from $500 to $4. Simvastatin (Zocor) dropped from $200 to $5. These aren’t outliers. They’re the rule.Same rules, same standards
Some people worry generics aren’t held to the same standards. They’re not. The FDA inspects every manufacturing facility - brand and generic - the same way. Every batch of generic pills must meet the same quality, purity, and potency standards as brand-name drugs. The FDA does over 12,000 inspections a year around the world. Generic manufacturers must prove their drugs stay stable for 12 to 24 months. They must maintain potency within 90-110% of what’s on the label. That’s identical to brand-name requirements. The only differences you might notice? The color, shape, or flavor. Those are just for branding. They don’t affect how the drug works. In fact, trademark laws prevent generics from looking exactly like the brand-name version. That’s why your generic pill might be white and oval instead of blue and capsule-shaped. But inside? Same medicine.Who’s saving money - and how much
In the U.S., generics make up 90% of all prescriptions filled. But they account for only about 18% of total drug spending. That means brand-name drugs - which make up just 10% of prescriptions - cost 82% of the total. That’s a $293 billion annual savings for the U.S. healthcare system, according to the Association for Accessible Medicines. From 2007 to 2016, generics saved Americans $1.67 trillion. That’s money that went back into people’s pockets, insurance premiums, and hospital budgets. Most insurance plans treat generics as Tier 1 drugs. That means a $0 to $15 copay. Brand-name drugs? Often $25 to $50. Specialty drugs? You might pay 25% to 33% of the cost. That’s why pharmacists automatically substitute generics unless you or your doctor specifically ask for the brand.
When people hesitate - and why
Despite the evidence, some people still don’t trust generics. A 2023 Tebra survey found that 62% of Americans say they trust brand-name drugs more. But 84% also admit generics are just as effective. That gap? It’s mostly about perception. Some patients report feeling different after switching. Maybe they had a headache. Or felt more tired. But in most cases, these are placebo effects - or unrelated changes. The FDA has documented cases where patients confuse a new pill’s appearance with a change in effectiveness. That’s why pharmacists now spend 3 to 5 minutes counseling patients on new generics, explaining the differences in look and reassuring them about the medicine inside. There are rare exceptions. For drugs with a narrow therapeutic index - like warfarin, levothyroxine, or phenytoin - even tiny changes in blood levels can matter. Some doctors prefer to keep patients on the same brand or generic manufacturer to avoid any possible variation. But even here, the FDA says all approved generics meet the same standard. If you’re concerned, talk to your pharmacist. They can help you track which generic you’re on and when it changes.The future of generics
More brand-name drugs are losing patents every year. In 2023 alone, over 150 drugs with combined sales of $157 billion are set to go generic. The FDA is speeding up approvals for complex generics like inhalers and ointments, which used to take years. New initiatives aim to cut approval times in half. The biggest challenge? Supply chains. Over 70% of the active ingredients in generics come from just two countries: China and India. Pandemic-related disruptions in 2020-2022 caused shortages of 287 generic drugs. That’s a risk. But it’s also pushing the U.S. to invest in domestic manufacturing. For now, the message is clear: generics work. They’re safe. They’re effective. And they save you - and the system - massive amounts of money.What to do next
If you’re on a brand-name drug, ask your doctor or pharmacist: "Is there a generic version?" If there is, it’s almost always the smarter choice. You’re not giving up quality. You’re just skipping the marketing bill. If you’ve had a bad experience switching to a generic, don’t assume it’s the drug. Talk to your pharmacist. Check if the manufacturer changed. Sometimes, a different filler or coating can cause minor side effects. But that doesn’t mean the medicine doesn’t work. And if cost is a barrier? Most pharmacies have discount programs. GoodRx, RxOutreach, and even manufacturer coupons can bring prices even lower. Generics are already cheap. With a little effort, they can be free.Are generic drugs as effective as brand-name drugs?
Yes. The FDA requires generic drugs to have the same active ingredient, strength, dosage form, and route of administration as the brand-name version. They must also prove bioequivalence - meaning they deliver the same amount of medicine into your bloodstream at the same rate. Studies show generics work just as well for the vast majority of patients.
Why do generic pills look different?
Trademark laws prevent generic manufacturers from making their pills look identical to the brand-name version. That’s why the color, shape, or size might be different. But those changes only affect appearance - not how the drug works. The active ingredient is exactly the same.
Can I trust generics made in other countries?
Yes. The FDA inspects all manufacturing facilities - whether in the U.S., India, China, or elsewhere - using the same strict standards. Over 12,000 inspections are done annually worldwide. If a facility doesn’t meet U.S. quality standards, the FDA blocks the product from entering the country.
Why do some people say generics don’t work for them?
Sometimes, it’s psychological - noticing a change in pill appearance can make people feel like something’s wrong. In rare cases, switching between different generic manufacturers (with different inactive ingredients) might cause minor side effects. For drugs with a narrow therapeutic index, like levothyroxine, some doctors prefer consistency. But the FDA confirms all approved generics meet the same safety and effectiveness standards.
Does insurance cover generics better than brand-name drugs?
Yes. Most insurance plans have three tiers: Tier 1 (generics) costs $0-$15, Tier 2 (brand-name) costs $25-$50, and Tier 3 (specialty drugs) can cost 25-33% of the price. Pharmacists can often switch you to a generic automatically unless your doctor says otherwise.
Are there any drugs that don’t have generics?
Yes - but they’re rare. New drugs are protected by patents for 20 years. Some complex drugs, like biologics (used for cancer or autoimmune diseases), take longer to develop generics for. These are called biosimilars and are just starting to become available. But for most common medications - blood pressure pills, antidepressants, statins - generics have been around for years.





Tom Costello
December 2, 2025 AT 23:08Generics are the unsung heroes of modern medicine. I’ve been on atorvastatin for years now-same results, no side effects, and I save $450 a month. My pharmacist even gave me a free pill organizer when I switched. Why pay more for a logo?
Also, the FDA inspections on foreign plants are way stricter than most people think. I worked in pharma compliance for a decade. They’ll shut down a facility over a speck of dust. No joke.
dylan dowsett
December 4, 2025 AT 18:32Okay, but-wait, hold on-I’ve seen people have panic attacks after switching to generics, and then they blame the drug, but it’s not the drug, it’s the *shape*! The color! The *labeling*! People are *so* suggestible! And don’t even get me started on how some pharmacies swap brands without telling you-no consent, no warning-just a different pill in the bottle! This is terrifying! And nobody’s talking about it! Someone should sue!
Wendy Chiridza
December 4, 2025 AT 23:01I switched my mom to generic levothyroxine last year and she had zero issues. She’s 72, has been on it for 15 years, and her TSH is perfect. The pharmacist spent 5 minutes explaining the difference in appearance and she was fine. Honestly, the fear around generics is mostly cultural. We’re conditioned to equate price with quality. But medicine isn’t wine.
Also, I love that the FDA inspects every facility the same way. That’s the kind of oversight we need more of.
Pamela Mae Ibabao
December 6, 2025 AT 17:32Let me just say this: if you’re still paying full price for brand-name statins, you’re being scammed. Not by the pharmacy. Not by the doctor. By your own brain. You think you’re getting ‘better’ medicine because it has a fancy name? Nah. You’re paying for the ad jingle. The celebrity endorsement. The fancy packaging. The placebo effect. And guess what? Your body doesn’t care what color the pill is.
Also, the fact that generics save $293 billion a year? That’s not a number. That’s real people keeping their homes. Paying for their kids’ school. Not choosing between insulin and groceries. So yeah, I’m mad you’re still hesitating.
Gerald Nauschnegg
December 7, 2025 AT 18:26Wait, so you’re telling me I could’ve been saving $500 a month for the last 5 years? I’ve been on Lipitor since 2018. I thought the generic was sketchy. I even asked my doctor twice if it was ‘the same.’ He said yes. I still didn’t switch. I’m an idiot. I just thought big pharma wouldn’t let a $4 pill exist. Turns out, they’re just greedy. And I paid for it. Thanks for the reality check.
Joanne Rencher
December 8, 2025 AT 04:04Ugh, I hate how Americans think everything’s a scam unless it’s expensive. We’re so gullible. In the UK, generics are the default. No one even thinks twice. You get the pill, you take it, you live. No drama. No ‘is this real?’ nonsense. We don’t need a 2000-word essay to understand that a pill with the same active ingredient does the same thing. It’s science, not a cult.
Erik van Hees
December 8, 2025 AT 20:27Actually, there’s a hidden catch. The FDA allows generics to vary by up to 20% in absorption rate. That’s not a typo. 20%. For most drugs, fine. But for someone on warfarin or thyroid meds? That’s a big deal. And no one tells you that. The FDA says it’s ‘clinically insignificant’-but that’s a bureaucratic way of saying ‘we hope you don’t die.’
Also, did you know that 70% of the active ingredients come from China? And China has zero transparency on their environmental standards? You’re literally ingesting pollution. You think your pill is safe? It’s made in a factory that dumps waste into the Yangtze. That’s not medicine. That’s a gamble.
Cristy Magdalena
December 9, 2025 AT 10:22I switched to generic omeprazole and I swear I felt like I was dying for two weeks. My stomach was on fire. My head was pounding. I thought I was having a heart attack. I went to the ER. They said it was ‘probably the change in inactive ingredients.’ But I didn’t trust it. I went back to Prilosec. I paid $300 a month. I cried. I felt like a sucker. But I felt better. And now I’m scared to switch again. What if it happens again? What if next time it’s worse? I don’t want to risk my life for $6.
Why doesn’t anyone just let us choose? Why is it always ‘you should be grateful’? I’m not ungrateful. I’m terrified.
Adrianna Alfano
December 10, 2025 AT 15:55Thank you for writing this. I’m a nurse and I see this every day. Patients cry because they can’t afford their meds. Then they hear ‘there’s a generic’ and they get this look like they’re being told to just ‘suck it up.’ But it’s not that simple. Some people have trauma around pills. Some had bad reactions. Some are scared because their grandma died on a generic in the 90s and no one ever explained why.
So when I talk to them, I don’t just say ‘it’s the same.’ I say ‘I get why you’re scared. Let’s look at the label together. Let’s call your pharmacist. Let’s make sure you’re not alone in this.’
And yeah, I’ve seen people switch and feel better because they stop stressing about the cost. That’s real healing too.
Casey Lyn Keller
December 12, 2025 AT 02:21So let me get this straight. You’re telling me the entire U.S. healthcare system is built on a lie? That we’ve been paying billions for marketing? That the FDA is just letting China and India make our pills? And nobody’s been held accountable? This feels like a cover-up. I’m not saying the generics don’t work-I’m saying someone’s making a fortune off our fear. And you’re just here saying ‘trust the system.’ No thanks. I’m sticking with the blue pill.